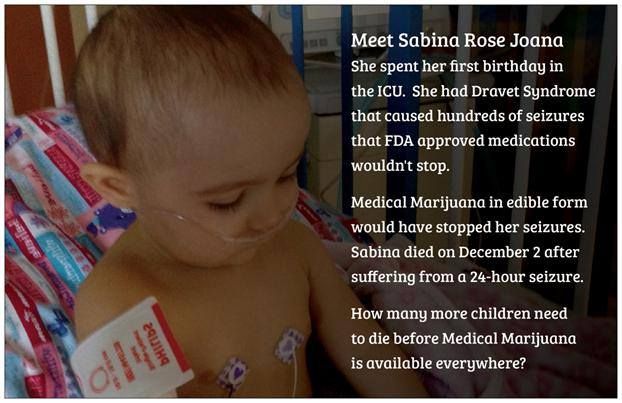
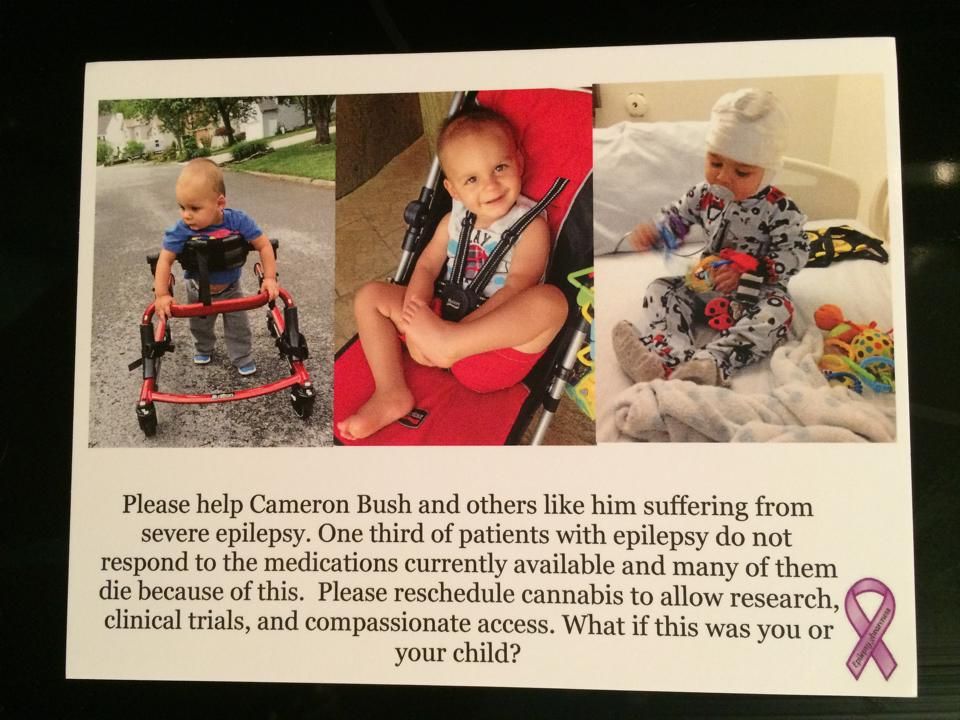
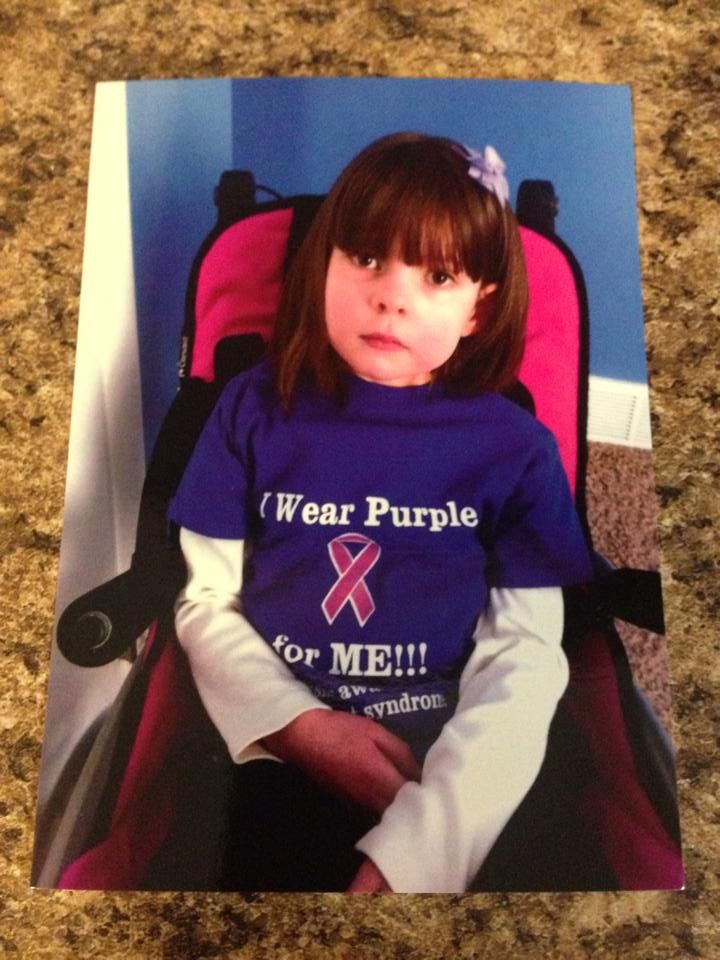
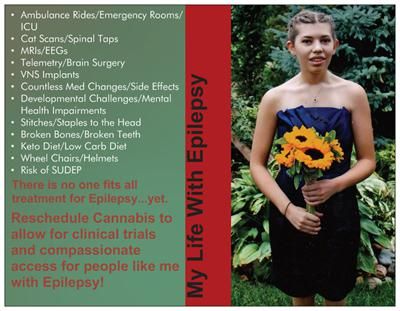
This is an interview with cannabidinoid researcher, Pritesh Kumar. Mr. Kumar earned a B.S in Biology at The University of Kentucky and a M.S. in Pharmacology at The University of Louisville. In 2014, he will earn his PhD in Pharmacology from The University of Louisville. Since 2009, Pritesh has been a research scientist at The University of Louisville Department of Pharmacology and Toxicology. In 2013, he joined Quantum 9, Inc. Cannabis Consulting and Technology.
Suzanne:
Kentucky is fortunate to have a scientist like yourself, with serious interest in the endocannabinoid system and cannabinoids, hailing from our state. Please tell us about your interest in the subject and the research you have done thus far.
Pritesh Kumar:
I have always been fascinated in the endocannabinoid system for several reasons. The way this system modulates and regulates our homeostasis is truly breathtaking. Name any condition or pathology with an inflammatory component, and 95 % of the time the endocannabinoid system is found to play some sort of role. Even from an evolutionary perspective, it is truly amazing that this system has been found in many phylogenetically diverse organisms.
I apologize for the digression. So, our research currently focuses on how ligands (chemical compounds) bind and interact with the cannabinoid receptor 2 (CB2). This receptor is predominantly localized in the peripheral system in immune cells, T cells, B cells, etc. The reason why this receptor is an attractive therapeutic target is because of it’s minimal presence in the central nervous system (CNS). Therefore, any medication targeting this receptor will not elicit the “high.”
Recently, we have been testing the current FDA-approved medications on the market to determine if they have any effects on the endocannabinoid system. As I mentioned previously, we found that a commonly prescribed drug for osteoporosis in post-menopausal women (Raloxfiene) is acting on the CB2 receptor. This bring us to an important point. Patients should have access to all the information of the drug they are taking. For example, it is well known that Raloxifene acts through the estrogen receptor to produce its effects. However, we found that it also interacts with CB2. Now, this interaction could help explain some of its known therapeutic effects or it’s mechanism of action. Furthermore, this drug could be “repurposed” for a different indication for which CB2 is a target (pain, inflammation).
Suzanne:
Since your PhD is in pharmacology you would be a great person to address a specific concern of many medical cannabis advocates. Various compounds in the plant appear to work together in an entourage effect to produce medicinal benefits. Many of us are concerned that once they start
isolating these compounds in labs and producing them synthetically for drugs that might not work as well as full plant and there will be more side effects. Are these concerns warranted?
Pritesh Kumar:
These concerns are warranted. From a pharmacology perspective, it is attractive to isolate a single component and market that as most drugs on the market have a single active ingredient. However, the cannabis plant displays a rather unique pharmacology. Plant-derived cannabinoids often work in concert to produce what is known as the "entourage effect." In this sense, a defined concentration or mixture of cannabinoids is necessary to reach therapeutic effect. For example, Sativex is marketed in Canada for the treatment of multiple sclerosis and this drug contains a 1:1 mixture of THC to CBD.
As to your second question regarding if more side effects will be produced if only the synthetic form is available and marketed. This a bit of a grey area. For example, synthetic cannabinoids are already out on the market and are available at gas stations and corner stores in the form of "K2 or Spice." These synthetic cannabinoids (mainly JWH derived) are very dangerous and consumption has led to a rise in visits to the ER because the compounds in these products are chemical analogues of THC are significantly more potent than THC. Unlike THC which is a partial agonist, these compounds are full agonists for the cannabinoid receptor 1 (CB1).
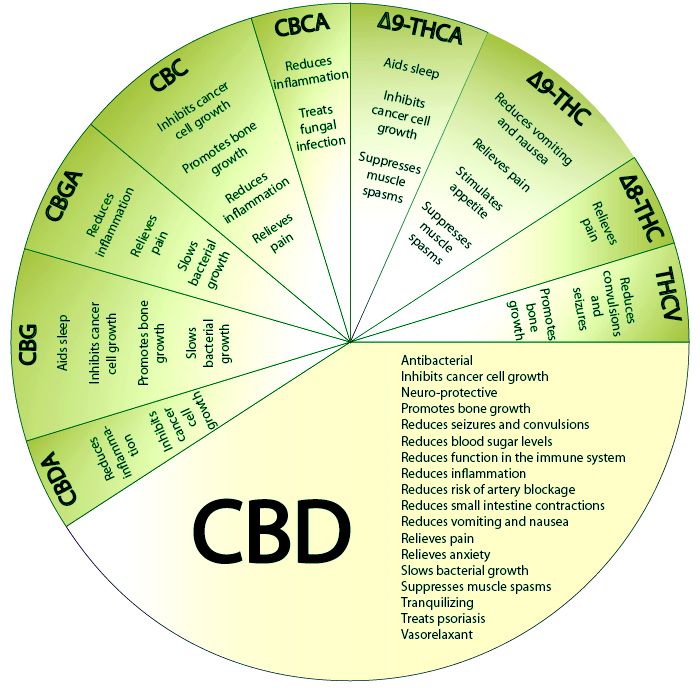
From a pharmacological perspective, things get complicated when you have to predict how multiple cannabinoids will effect the body. It is often easier to investigate a single component or compound rather than a mixture. That being said, for the cannabis plant, there are multiple layers that often contribute to the beneficial effect of this plant (THC, CBD, CBG, CBN, etc). To answer your question, we still do not know if synthetics will have a worse side effect profile compared to the plant.
Suzanne:
What is next in terms of your research? In what direction are you looking once your PhD is completed this year?
Pritesh:
I am considering several options at the moment. I will continue to do research but from a slightly different perspective. I am personally interested in the plant cannabinoids as they may hold tremendous therapeutic benefit and represent an untapped source of potentially novel medications. I will stay on as consultant for Quantum 9, Inc. which is one of few companies out there that are legitimately interested in helping patients in the long run. Personally, I envision operating a company with a research facility in the state of Kentucky which will be heavily focused on the development of medications from plant cannabinoid extractions and perform laboratory testing for cannabis samples once this bill is passed.
Suzanne:
Is there anything else you would like to say to our readers about the endocannabinoid system, cannabinoids, and the future of medicinal cannabis?
Pritesh:
To the readers, I want to say that the endocannabinoid system is a beautiful network of receptors and enzymes that regulate a broad spectrum of signaling pathways in our bodies. The future of cannabis-based medications is still in its infancy and the rate at which we are developing these types of medications is rapidly increasing.
Pritesh Kumar
Cannabinoid Research Scientist
Quantum 9, Inc.
351 W Hubbard Suite 303 Chicago, IL 60607
888.716.0404 ext 809
www.quantum9.net
LinkedIn|
Pritesh Kumar's Peer Reviewed Publications:
1) Kumar A, Qiao Z, Kumar P, Song ZH. (2012). Effects of Palmitoylethanolamide on Aqueous Humor Outflow. Invest Ophthalmol Vis Sci. Accepted doi: 10.1167/iovs.11-9294
1.Kumar A, Qiao Z, Kumar P, Song ZH. (2012). Involvement of a non-CB1/CB2 cannabinoid receptor in the aqueous humor outflow-enhancing effects of abnormal-cannabidiol. Exp Eye Res. Accepted
http://dx.doi.org/10.1016/j.bbr.2011.03.031
2.Kumar P, Song ZH. (2013). Identification of raloxifene as a novel CB2 inverse agonist. Biochem Biophys Res Commun. doi: 10.1016/j.bbrc.2013.04.040. [Epub ahead of print]
3. Kotsikorou E, Navas F 3rd, Roche MJ, Gilliam AF, Thomas BF, Seltzman HH, Kumar P, Song ZH, Hurst DP, Lynch DL, Reggio PH (2013).The importance of hydrogen bonding and aromatic stacking to the affinity and efficacy of cannabinoidreceptor CB2 antagonist, 5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)methyl]-N-[(1S,2S,4R)-1,3,3-trimethylbicyclo[2.2.1]hept-2-yl]-1H-pyrazole-3-carboxamide (SR144528).
4. Kumar P, Song ZH. (2013). Structure-activity relationships of fatty acid amide ligands in activating and desensitizing G protein-coupled receptor 119. Eur J Pharmacol. doi: 10.1016/j.ejphar.2013.10.044.
Kumar P, Song ZH (2013). CB2 cannabinoid receptor is a novel target for third-generation selective estrogen receptor modulators bazedoxifene and lasofoxifene. Biochem Biophys Res Commun. Accepted.
1.Kumar P, Carrasquer C, Carter A, Song ZH, Cunningham AR (2014). A categorical structure-activity relationship analysis of GPR119. Journal of Molecular Modeling. In press.